Case Studies
Open our summaries below to learn about how we help our clients save money, launch on-time and improve their products.
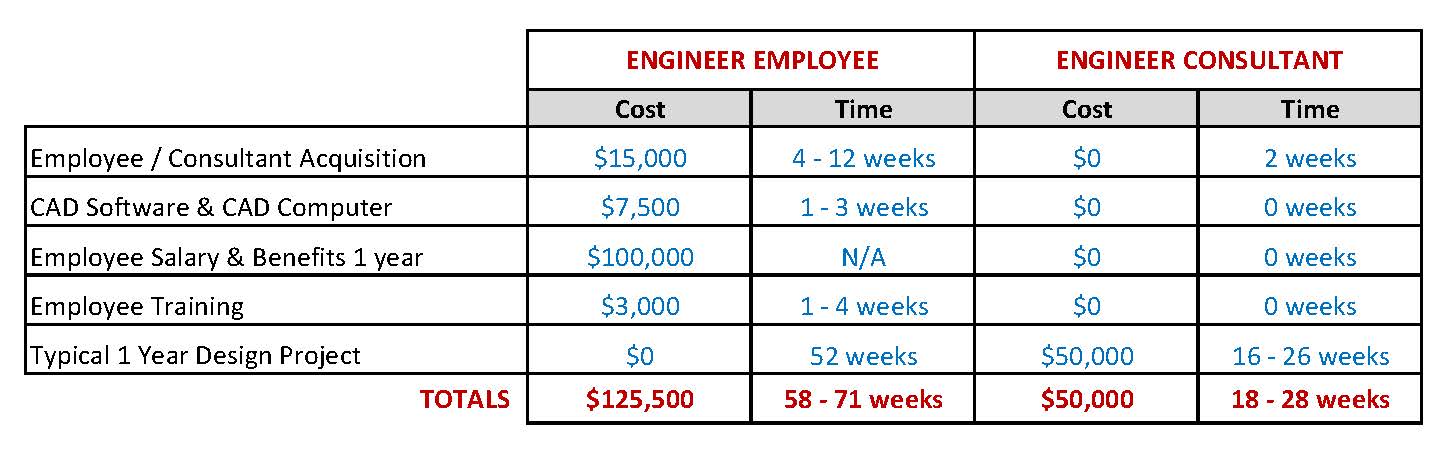
Using Outsourced Engineering: Do the Numbers Add Up?
If you’re thinking about adding design engineering resources for a development project, first check out this comparison of the impact of hiring an employee versus using outsourced development resources.
The following example shows the typical costs and time for finding, hiring, training and integrating a new staff engineer into an organization and for engaging outside design resources when needed.
Benefits of Outsourced Resources from EQS:
- Experts in Medical Device design & manufacturing
- Current on industry technology, trends, standards and regulations
- Ready to work when needed
- Provide complete, clean and organized models and drawing packages
- Maintain a network of resources to help projects succeed
- Professional & prompt responses
- All computers, software, tools included
Product Development Outsourcing may be the answer for your new project! Contact us today to find out more.
About half-way through a new project, an orthopedic device company lost their key development engineer. In order to avoid a costly delay, the company contracted with EQS to complete the designs, documentation, validation and verification tasks and get their products ready for regulatory submissions and manufacturing. Our expertise in medical device development helped them seamlessly complete the project on-time and under budget, while complying with applicable FDA design requirements.
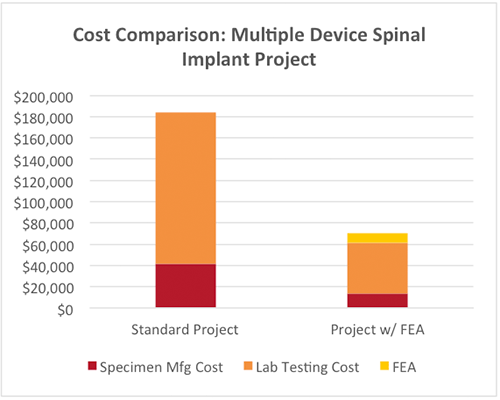
Three different IBFD spinal implants were developed in parallel paths. With regulatory input, FEA was performed to simulate laboratory testing and determined the weakest designs across all three product families in each of three test modes.
As a result, specimen manufacturing and testing costs were reduced by nearly two-thirds, and timelines were accelerated by two months.
Mechanical testing was completed on an IBFD device consisting of PEEK and titanium components. After test results fell short of acceptance thresholds, EQS conducted an in-depth analysis of the design, test samples and data. Using computer simulation and our knowledge of spinal device testing and function, we determined the causes of the device failure and completed design modifications and improvements. The new design performed significantly better than previous versions and test results met substantial equivalence requirements, leading to a successful 510(k) submission.
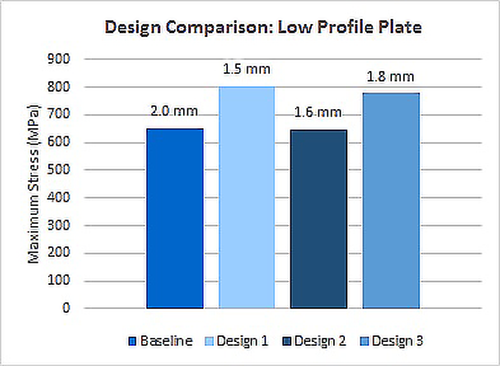
A small fragment plate was under development where a low profile design was critical to create a competitive advantage. The initial design was manufactured and mechanically tested to failure and a FEA model was created and validated to match laboratory test data.
Using the validated FEA model, several design iterations were compared in order to optimize device geometry, material, and strength, resulting in a superior product. The final design was 20 percent thinner than the baseline with approximately the same strength and performance.


